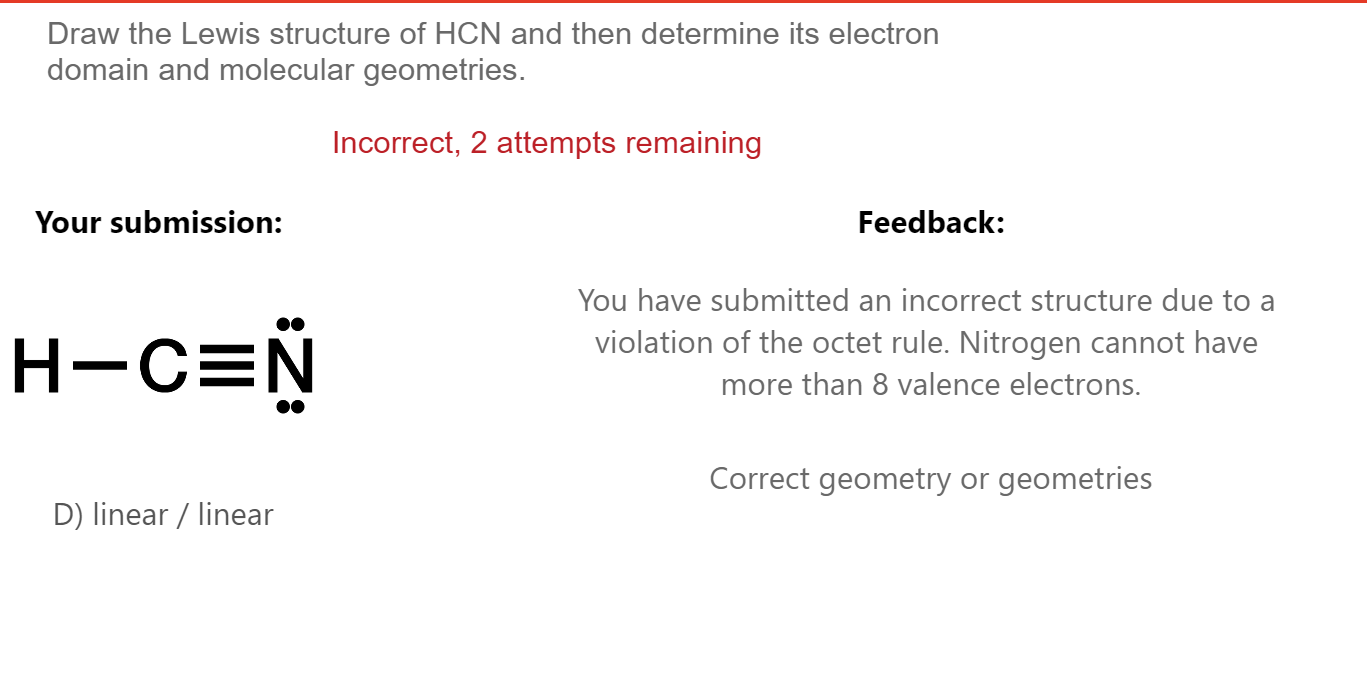
Draw The Lewis Structure Of Hcn
Draw The Lewis Structure Of Hcn - The hcn molecule consists of three atoms: H + c + n =1 + 4 + 5 = 10. Put least electronegative atom in centre 3. Here's how to do it. Web =1+4+5 = 10 valence electrons. For the hcn lewis structure, calculate the total number of valence electrons for the hcn molecule. This molecule is made up of three different atoms: How to draw the lewis structure for hcn. Put the least electronegative atom c in the middle with h and cl on either side. But serious, it’s dangerous, so stay away.
The lewis structure of hcn shows the arrangement of atoms and electrons in the molecule. And does it exhibit resonance? Web to draw the lewis dot structure of any molecule, it is essential to know the total number of valence electrons in the structure. Hydrogen (h), carbon (c), and nitrogen (n). Here's how to do it. Ch 4 has 4 valence electrons in c, and 1 in each of the four h: There are 2 steps to solve this one.
Web to sketch the hcn lewis structure by following these instructions: Establish a general procedure for drawing lewis structures. You nave to put a triple bond between c and n. Hcn is a linear molecule, since it has only two electron groups and there are no lone pairs on the central carbon atom. To know the valence electrons of hcn, let us go through the valence electrons of individual atoms in hydrogen cyanide.
But serious, it’s dangerous, so stay away. Web the lewis structure of hcn explains that the hydrogen atom has one valence electron and it is a donor atom, the carbon atom has four valence electrons as it is from the 14th group of the periodic table and the nitrogen atom has five electrons. Hcn lewis dot structure by counting valence electrons on the carbon and nitrogen atom. Establish a general procedure for drawing lewis structures. Now you can see that the central atom here is carbon because it is easy for carbon to become stable as it is the least electronegative of all. There is 1 lone pair on the nitrogen atom (n).
To know the valence electrons of hcn, let us go through the valence electrons of individual atoms in hydrogen cyanide. Web draw out a correct lewis structure for the following compounds. H + c + n =1 + 4 + 5 = 10. Is the lewis structure correct? What is this molecule and what is it used for?
Web your instructions for only $21/task. What is this molecule and what is it used for? Web draw the lewis dot structure of hydrogen cyanide (hcn) molecule. Put one electron pair in each bond 4.
H + C + N =1 + 4 + 5 = 10.
For the hcn lewis structure we have one valence electron for hydrogen, we have four for carbon, and we have five for nitrogen, for a total of ten valence electrons for the hcn lewis structure. Web draw the lewis structures of ch 4, pcl 3, co 2, and hcn. You nave to put a triple bond between c and n. 3.8k views 6 years ago chem 101:
Use These Steps To Correctly Draw The Hcn Lewis Structure:
Web to sketch the hcn lewis structure by following these instructions: Lewis structure of hcn for counting valence electrons around the terminal hydrogen atoms. Hydrogen (h), carbon (c), and nitrogen (n). Hcn is a gas used primarily in chemical synthesis, mining, and polymer manufacturing.
6 Steps To Draw The Lewis Structure Of Hcn.
Put least electronegative atom in centre 3. Count the valence electrons you can use. Hcn, hydrogen cyanide, is rather poisonous. How to draw the lewis structure for hcn.
Is The Lewis Structure Correct?
There are 2 steps to solve this one. Pcl 3 has 5 valence electros in p and 7 in each of the three cl: = 5 + 7x3 = 26 valence electrons Put the least electronegative atom c in the middle with h and cl on either side.